
Automation of critical business processes
In this article, we will analyse how process automation makes it possible to control processes, generate reliable information to generate robust product traceability and facilitate decision-making, as well as process optimization depending to a lesser extent on plant users.


GxP Principle
When implementing computerized systems in regulated sectors such as pharmaceuticals or healthcare, the existence of regulations applicable to the verification and maintenance of computerized systems must be taken into account. The purpose of these standards is to ensure the intended use of the systems throughout their life cycle, i.e. from their acquisition or development, through the verification of their operation, assurance throughout their period of use, to their retirement.
Annex 11 of the GMP, dedicated to computerized systems, states as a premise that “where a computerized system replaces a manual operation, it should not be to the detriment of product quality, process control or quality assurance. There should be no increase in the overall risk of the process“.
This premise must be kept in mind throughout the entire automation process. A very effective way to comply with it is to always bear in mind the needs of the process to be automated. In other words, prior to the acquisition or development of the systems to be implemented, the functional, security or data integrity requirements of the process must be analysed. In this way, the planned coverage will be offered both in terms of process control and traceability.
Focusing on the characteristics and requirements of the processes to be automated and not on the standard operation of the computerized systems to be implemented will ensure part of the success of the project.
Objectives of automation
In the following, the objectives of automation are analysed from different perspectives:
Continuous improvement
A widespread practice in industry is lean manufacturing. This methodology is based on the exhaustive knowledge of processes, focusing on the knowledge and experience of its specialists: the people who design, execute and control them.
With this knowledge of the process, actions are designed to eliminate process risks, generate accurate information and eliminate waste (everything that does not generate value).
Lean manufacturing, therefore, generates an environment that constantly seeks to optimize and control processes, and valuable information is the foundation of this strategy. A motto of this methodology is “to improve, you must first measure“.
One of the ways to achieve this valuable information is through the use of computerized systems that monitor and record data in real time.
Process Assurance
Until relatively recently, the industry controlled its critical processes by means of procedures that clearly and precisely described the management to be carried out by the operators involved and the record required to record the execution and the values of the critical parameters obtained in the course of the process. This management was considered to be complete and correct, however, it has risks intrinsic to its design. The main risks of manual process management are the high dependence on the operator to execute the tasks at the right time, in the right way and of the right quality, as well as the risk associated with the manual recording of the process data by the user.
Implementing computerized systems for the control of critical processes brings the following advantages:
- Automatic recording of information and avoiding, as far as possible, manual data entry by the operator. For example, real-time automated recording of the date and time, the operator performing the action, the product used (by barcode scanning or similar), as well as process values through integration with process equipment (such as production batchers, scales or climates) or laboratory instruments.
- It allows the operator to be guided in their tasks, to follow the protocols defined and in force, and to introduce in-process controls to avoid errors. An example of this management is to determine the task to be performed by a warehouse operator, guiding the operator in the preparation of the materials of the highest priority order according to planning and specifying the warehouse slot and the package to be prepared in order to optimize their movements. This management also makes it possible to avoid errors, for example, by ensuring that the user has prepared the planned batch of product by requesting confirmation of the package by reading the barcode.
- Automatic representation of the information. The automatic recording of the process makes it possible to exploit the information both for decision-making and to support the operators who are executing or monitoring the process. The information treatment graphs grouped in the room allow observing trends that can support the user in the control of the process, to avoid incidences, as well as to monitor the fulfilment of objectives.
- Management of alerts and notifications.
The automation of critical business processes is becoming increasingly important for companies in the healthcare, pharmaceutical, cosmetics and food industries.
Real-time and automated process management allows notifications to be sent to users to inform them that certain events have occurred in a specific context. This management can allow a very important time optimization, as well as avoiding product quality incidents and avoids the dependence of the users on the process, allowing them to concentrate as much as possible on the management of the process itself.
Regulatory compliance
The regulations applicable to the regulated sectors are becoming increasingly demanding, requiring the execution of in-process controls and the recording of their evidence. This requirement is finally passed on to the plant operator, who is responsible for recording the values of the process, asking him to carry out increasingly more frequent calculations, sometimes very complex, which may increase the risk of error in the calculation or recording of the result, as well as the operator shifting his attention to the calculation and not to the process he is monitoring.
Process automation allows automatic data processing while avoiding human error and having the possibility to take into account a larger number of variables.
Data exploitation
Paper-based process management allows the recording of critical information to control the GxP process. But in the event of an incident or to have a grouped view of what happened, access to this information can be costly in time and form, as well as introducing certain risks.
Process automation allows the automated recording of critical variables. This recording allows the exploitation of information for decision making. It allows the definition of a strategy for action based on data. As well as to analyse the information taking into account different perspectives.
Risks of automation
Como se ha analizado en el punto anterior son numerosas las ventajas de la automatización de procesos, pero también tiene asociados riesgos que debemos destacar:
As discussed in the previous section, there are many advantages to process automation, but there are also risks associated with it that should be highlighted:
- Failure of the system to perform as intended. Uncontrolled and unsafe execution of the system.
- Unreliable data due to the absence of control measures. Throughout the data life cycle from generation through selection, representation, processing, storage, retrieval, distribution, use, archiving and destruction.
- Incorrect management of system maintenance and/or configuration.
- Incorrect grouping of information may result in an incorrect value and meaning of the indicator.
Types of computerized systems
During the automation process, use can be made of different types of computerized systems operating at different levels or for different purposes. Figure 1 identifies these levels of process and information management:
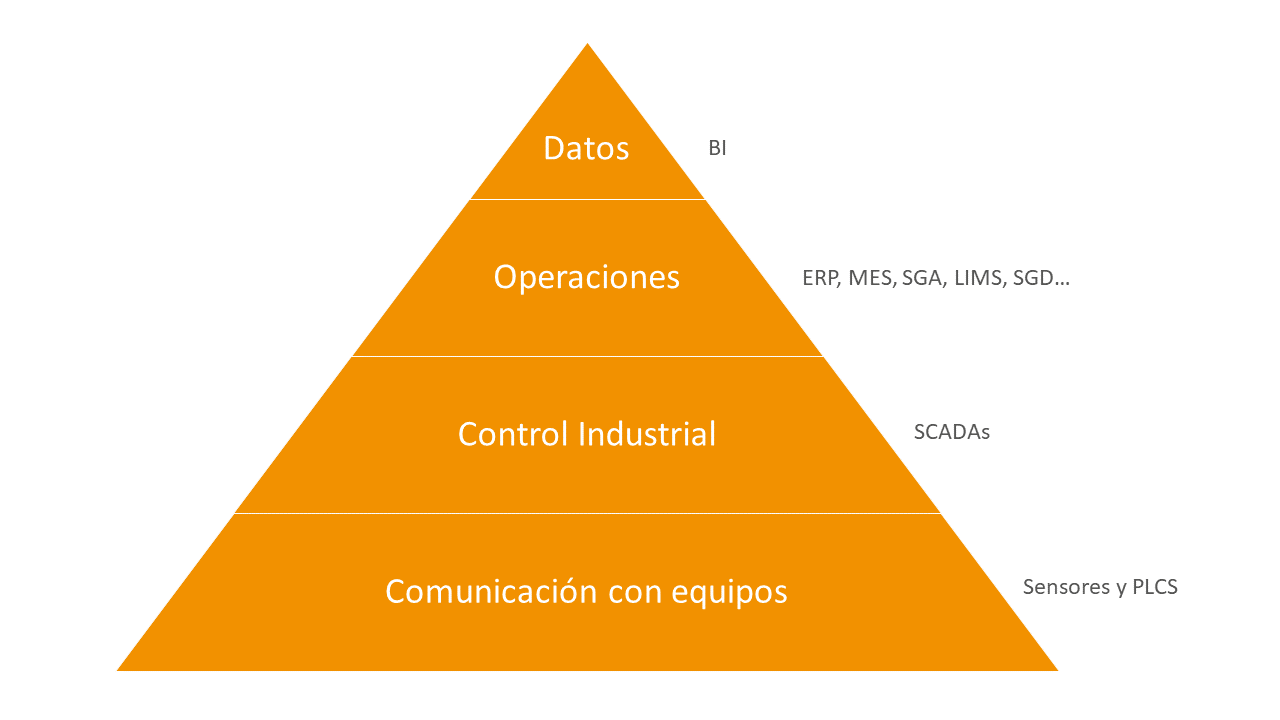

The first layer of the pyramid, starting at the bottom, refers to very low-level computerized systems that are able to integrate and understand signals from the plant’s physical/industrial equipment.
The second layer refers to industrial control. These systems make it possible to govern the physical plant equipment and manage the intervention of users in the process.
The third layer refers to operations. At this level, defined and regulated processes can be managed, taking into account user intervention, automating and controlling processes and integrating with equipment to reduce manual data entry.
The fourth layer called data allows exploiting the information, giving it meaning and enabling the organization to make strategic decisions.
Short, medium and long-term objectives
The objective of automation in the short term is to control critical business processes by using the end-users’ computerized system as a guide in the process and applying the necessary control measures. As well as obtaining the primary process data in real time.
In the medium term, automation provides a set of processed and reliable data that has meaning (relevance, purpose and context) and is therefore useful for decision-makers by reducing their uncertainty. In the long term, considering the information generated by the system and the experience and knowledge of the users, it serves as a framework for generating a strategic action plan based on data.
Computerized systems implementation strategy
Special attention should be paid to the automation strategy. The end goals should be set from the beginning, but the way the project is developed should be strategically planned and sequenced.
Process automation has many advantages, but resistance to change in organizations, if not properly managed, can be a major risk factor for the development and success of the project.
Figure 2 depicts the value chain of a medicine manufacturing company. The value chain allows the company to be examined and divided into its most relevant strategic activities. Two types of areas are identified, primary and support areas:
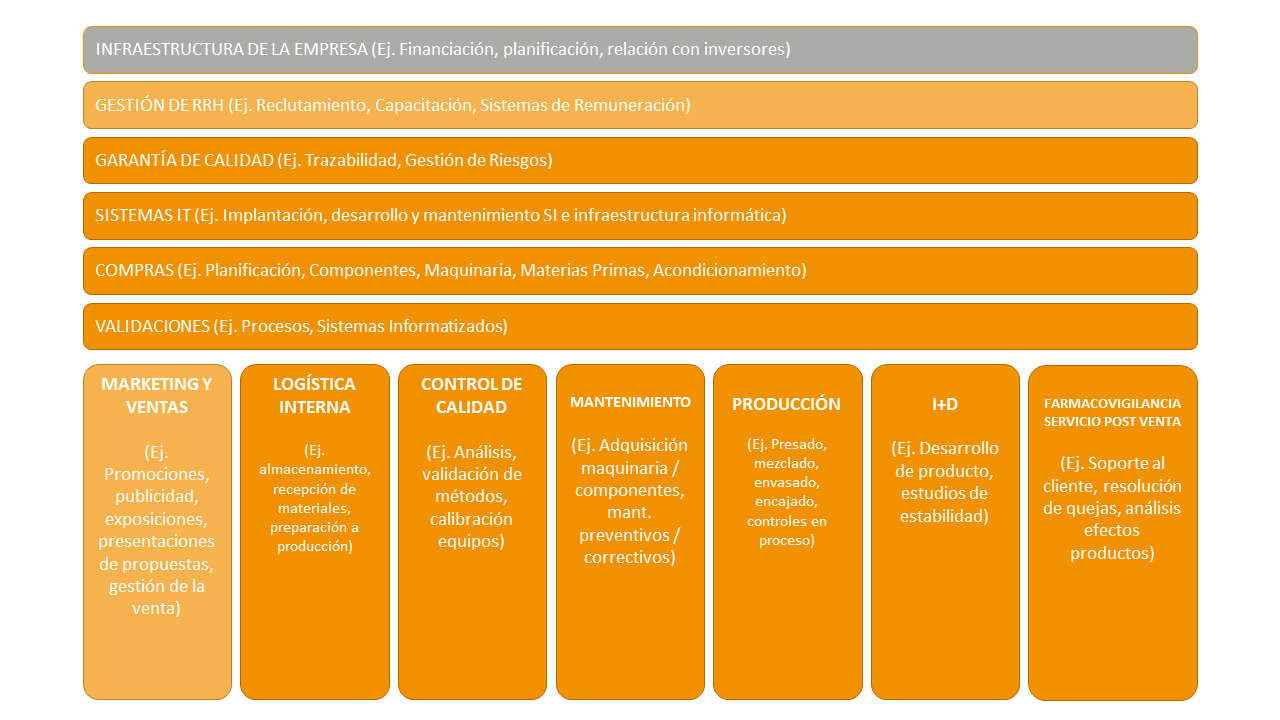

The primary areas are focused on the elaboration and/or control of the product and, although they depend on the rest of the areas of the organization, they manage their process individually. The main primary areas identified are production, logistics, quality control, R&D and pharmacovigilance.
Support areas can be understood as the departments that provide services to the primary areas for their correct development. The main support areas are quality, IT, human resources or purchasing.
To represent the value chain, a colour code has been used which identifies the degree of GxP impact of the areas represented. High GxP impact is represented by orange, medium GxP impact by a lighter orange and areas with no GxP impact are represented in grey.
Complete automation of areas and the organization is possible, but it is advisable to design a scalable system and to implement digitization projects for individual and controlled processes in the perspective of the complete digitization goal.
The automation process could be as follows:
- Start by implementing the basis of an ERP (enterprise resource planning system) for master data control (information on suppliers, customers, articles) and the administrative management of purchase and sales orders and the generation of production orders. The use of this system would be simple, but we would ensure that it is scalable when it comes to extending its functionalities and that it has the security required by the applicable regulations. Up to this point, we would have the basic information in digital form.
- From this point on, it would be advisable to continue with a warehouse management system. To manage in real time and in the facilities the entry of goods, the blocking of the quality of the materials, the supply of materials to production, the registration of the manufactured product and the dispatch of the products to customers. With this project, we could manage the stock properly, as well as control and register critical activities for the traceability and quality of the product.
- The next projects could be the automation of manufacturing, with an MES system, or the automation of the laboratory control process with a LIMS system.
- Management of support areas such as maintenance, with the implementation of a CMMS system, which controls corrective and preventive maintenance of process machines and installations. As well as documentation management with the implementation of a document management system or DMS.
- Having the information on stock, internal logistics, production, analysis and maintenance, a planner or MRP could be implemented to optimize times, machine capacities and stock management.
- Implementation of business intelligence, BI, for the exploitation of information for process control, decision-making and business strategy design.
The design of the computerized systems to be implemented will depend on the company’s digitalization requirements and objectives. If the company does not have a great complexity of processes, it could opt to select a global computerized system that has different modules for the management of the different processes, such as SAP or SAGE. Or if you have a more basic ERP and implement specialized computerized systems for certain activities, such as a warehouse management system, a laboratory management system, a document management system, etc.
Treatment of information
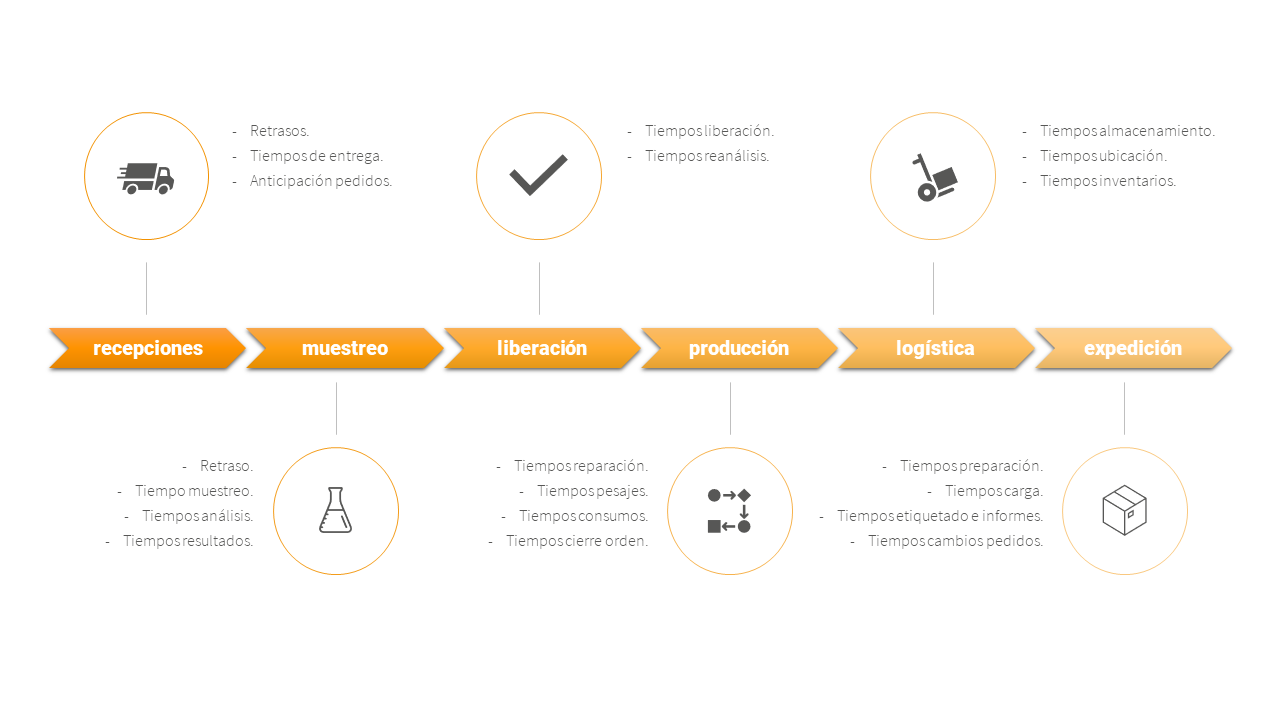

Figure 3 shows how the information can be exploited in a grouped form, giving an example of a time control study of the critical business processes of a drug manufacturing company. For each phase of a product’s life cycle, times to be monitored are identified. For each time parameter to be measured, an optimum time is established and compared with the actual time to determine possible deviations and investigate their root cause. In this way, corrective and preventive measures can be designed based on data.
Conclusions
- Process automation generates reliable information for decision-making by facilitating the execution of key processes for end users.
- Automation must be planned strategically by identifying the final objectives to be achieved, selecting the appropriate computerized systems and implementing the change in a phased manner so that it is digestible by the organization.
- Automation not only allows for proper traceability, but also enables the information to be exploited from different angles, allowing a data-driven strategic action plan to be designed.
Article originally published in Pharmatech magazine, issue 56 March-April 2021.
Do you need personalized advice for your company? Contact our consultants, without obligation, through our email info@oqotech.com or colombia@oqotech.com. We will be delighted to help you.








