
Withdrawal of Computerized Systems in GxP-regulated sectors


Normative Requirement
The good manufacturing practices include, as part of their activities, the control and verification of computerized systems. Any type of computerized system used as part of the activities regulated by the GMP requires validation.
In this sense, the main objective of the GMP is that when a computerized system replaces a manual operation, it should not be to the detriment of product quality, process control or quality assurance. There should be no increase in overall process risk.
Validation and its risk management should be applied throughout the life cycle of the computerized system, taking into account patient safety, data integrity and product quality. The life cycle is understood as all phases of a system’s life, from the initial requirements to its retirement, including design, specifications, programming, testing, installation, operation and maintenance.
Therefore, among the activities foreseen in the control and verification of computerized systems must be the withdrawal. This activity can sometimes be treated superficially, but it can be as critical a phase as the release or use of the system. An unplanned recall can jeopardize the historical information of the system and therefore the maintenance of product traceability.
In this article, we will discuss what we mean by a controlled recall of a computerized system in compliance with GxP and how to approach a complete, correct and documented recall plan.
Definition of the concept of withdrawal
The process of decommissioning computerized systems focuses on determining the final control and configuration of the computerized system to be decommissioned during its archival time and ensuring the integrity of its data, regardless of the associated processing equipment, if applicable.
It also takes into account the impact of the withdrawal on the management, control and documentation associated with the process currently managed by the computerized system to be withdrawn, as well as the possible impact on the organization’s internal staff, suppliers and contracted services.
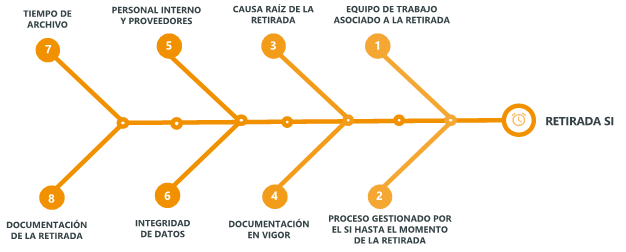
The following is a risk analysis in the form of a fishbone that identifies the main variables to control in this activity:

Description of the complete withdrawal flow
The tasks to be planned, executed and controlled within a GxP computerized system recall plan are identified below:
1. Definition of roles and responsibilities.
It is necessary to set up a working team to ensure the entire recall process, including key personnel of the organization. The team should include representatives of the affected processes, quality, systems and IT.
2. Description of the business process.
It is necessary to evaluate the current process that may be affected by the recall. We must ensure that we fully understand the scope of the system in managing the process, controls in place and records generated. This action helps to ensure that the impact of the recall is identified, allowing us to design a complete recall strategy, accounting for and mitigating all risks.
3. Impact of withdrawal.
Evidence that the effects of the impact of the withdrawal have been considered in aspects such as:
- The management, control and recording of the process currently governing the computerized system to be recalled.
- Identification of the root cause of retirement.
- Updating of procedures, documentation and records.
- Impact on internal and external personnel.
4. Anticipated future scenario.
It reflects the foreseen future scenario, identifying procedures or computerized systems to replace the current management. This definition makes it possible to require the necessary validations to approve the new computerized scenario.
5. Information management.
Planning for possible destruction, archiving or migration of data. Ensuring data integrity.
6. Verifications.
Ensures that documentation associated with the verifications is retained as part of the record and evidence of the recall.
7. System maintenance and support period.
Maintenance of the system during data archiving time. Evidence of control and definition of archiving time.
8. Change control.
It evidences the control of the established compliance of the withdrawal and that the results obtained are suitable and do not jeopardize the management and control of the process, nor the integrity of its data.
9. Planning.
Planning the recall process. Synchronizing: responsible, tasks, deadlines, sequences and control points.
10. Execution of the withdrawal.
Evaluation and determination of the moment of execution of the recall. They take into account the impact on the process, validation of new computerized systems and recall rollback strategy.
11. Documentation of the computerized system.
Ensures that the documentation associated with the computerized system to be retired is retained as part of the quality system.
12. Informed of withdrawal.
Generation of a final recall report, evidencing the activities carried out, results obtained, documentation and records generated and final recall report.
At OQOTECH we are experts, with more than 15 years in the sector, specialized in the validation of computerized systems. Contact our consultants and receive personalized advice for your company.








